The itch of a clothing tag. The seam on the inside of a sock. The tickling of hairs on the back of your neck. For many of us, it’s easy to tune out these sensations as we move through the day. But for some autistic people, everyday sensations can be intolerable.
David Ginty knows why, and it’s not, as many autism researchers once believed, a dysfunction of the brain.
Ginty, the Edward R. and Anne G. Lefler Professor of Neurobiology and chair of the Department of Neurobiology at Harvard Medical School, studies touch and pain. Scientists have known for some time, he said, that our experience of physical sensation is a collaboration between our brain, our central nervous system, and sensory neurons. But the mechanisms behind that collaboration have remained a mystery, and the quest for an answer has major implications for our ability to treat everything from chronic pain to autistic hypersensitivity to sexual dysfunction.
“The auditory system cares about sound waves in a particular frequency range,” Ginty said. “The visual system, similarly, only cares about a narrow band of the visual light range. But the somatosensory system cares about tactile stimuli, thermal stimuli, chemical stimuli, proprioception — where your body and limbs are in space and time, as well as the state of many of our body organs.
“And then there’s an affective component, an emotional component of touch, which in itself is a huge burgeoning area that is interesting. How does touch trigger an emotional response? Somatosensation is incredibly rich and multidimensional.”
“We’re looking hard to find non-opioid approaches to treat pain and we’ve identified many potential approaches.”
About 10 years ago, Ginty and his team found that in animal models of autism spectrum disorder, the locus of sensory dysfunction was not the brain, as had been thought, but the spinal cord and periphery. The key players are second-order neurons in the spinal cord that function like a mixing board’s gain or volume control, amplifying or dampening sensations as they travel from the skin and other sensory organs to the brain. In some ASD models, these second-order neurons appeared to be stuck on high, leading to sensory overload.
“It made us realize that we could potentially treat sensory over-reactivity by turning down the activity of sensory neurons, or sensory neuron responsiveness, in the peripheral nervous system,” he said.
The most logical approach would be to use drugs that turn down sensory neuron activity. Lauren Orefice, then a postdoc in the Ginty lab, thought that benzodiazepines could be used to silence nerve cells in the periphery to help reduce sensory over-reactivity. But pediatricians are reluctant to prescribe potentially addictive sedatives to their patients.
“So one approach that we’ve been trying to take is to develop peripherally restricted benzodiazepines that can reduce the activity of neurons in the peripheral nervous system without penetrating the brain, and therefore without sedating side effects,” Ginty said.
For children with autistic hypersensitivity, such a drug could be life-changing. It could reduce overstimulation, lower anxiety, prevent meltdowns, and let them experience a hug as a pleasure rather than a source of pain.
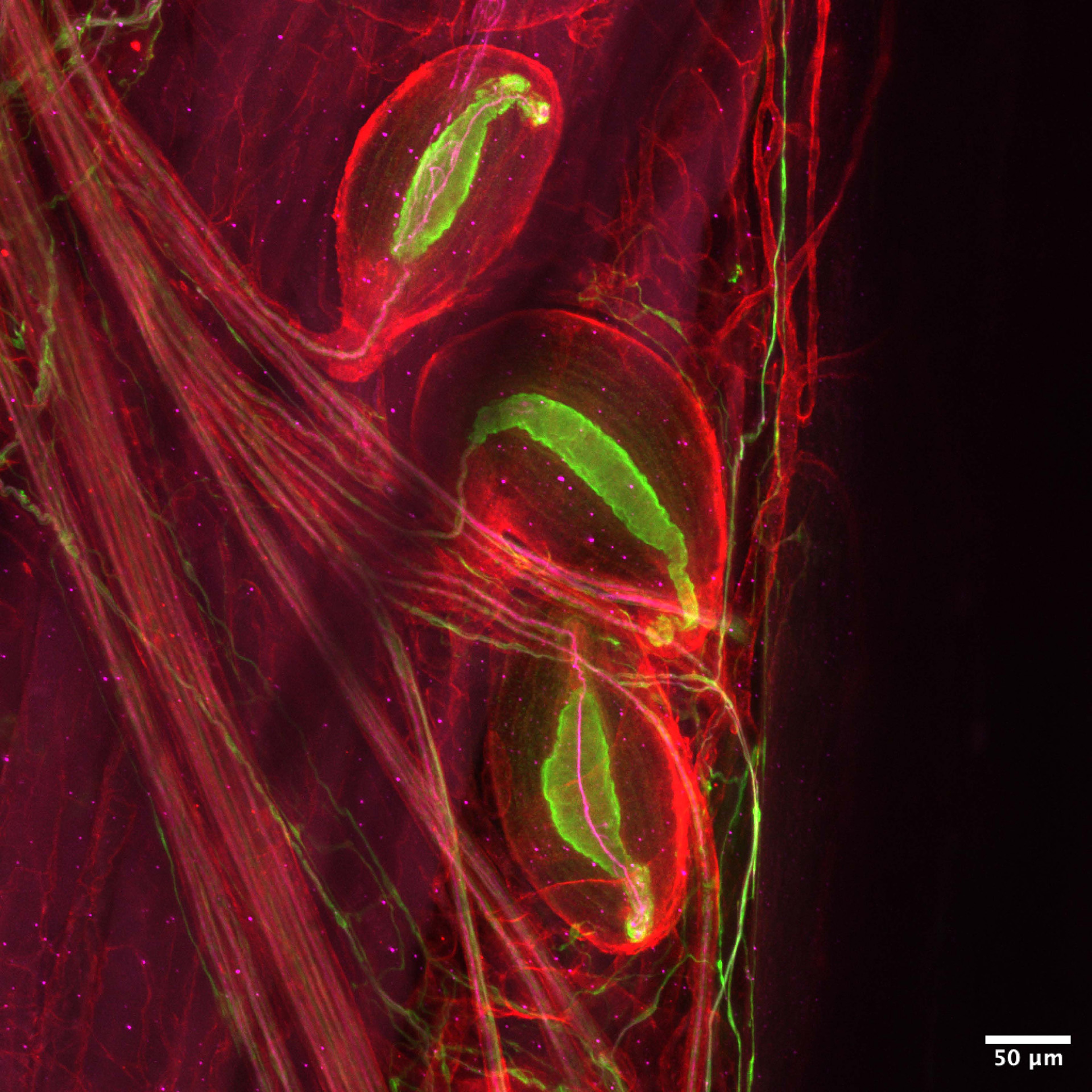
Pacinian corpuscles — neurons that sense vibrations — are delicate enough to pick up someone’s footsteps on the other side of the room.
Image by Zoe Sarafis
The implications of Ginty’s work on the systems underlying pleasure and pain extend far beyond autism research. The somatosensory system is made of some 20 types of neurons tucked into every imaginable part of the body: the base of our hair follicles, the crevasses of our dermis, in our muscles and joints — anywhere that detects variations of stretch, pressure, vibration, temperature, and even our position in space. If he had to pick a favorite neuron, he’d pick two: Pacinian corpuscles and nociceptors.
Pacinian corpuscles sense vibrations. They’re delicate enough to pick up someone’s footsteps on the other side of the room, and impactful enough to make us cry when music moves through our body. Nociceptors pick up on noxious stimuli — or, in plain English, pain.
“We’re figuring out how nociceptors are connected in the central nervous system to give rise to reflexes, like quickly removing your hand from a hot stove, or to the emotional component of pain,” Ginty said. “These are truly amazing neurons. They have very high thresholds, unlike the Pacinian corpuscles, which respond just to tiny tweaks or vibrations of the skin. The nociceptors only fire an electrical impulse when you have a damaging encounter.”
New genetic tools allow Ginty to understand how nociceptors connect to the central nervous system and identify every protein that nociceptors express, unlocking a new range of potential drug targets. “Right now, opioids are the best remedy we have for many types of pain, and that’s really gotten us into trouble,” he said. “We’re looking hard to find non-opioid approaches to treat pain and we’ve identified many potential approaches by targeting the nociceptors themselves.”
Ginty’s lab is not set up for drug development. But the research done in his lab forms the groundwork that the pharmaceutical industry needs to create treatments that improve lives. Ginty’s research is often exploratory, he said. It’s not always clear whether or how a certain experiment will translate into a therapy or marketable drug, which is why industry funding is rarely sufficient. It’s federal grants that have supported the fundamental science, which, in the long run, lead to cures.
Ginty has had two grants frozen in the Trump administration’s dispute with Harvard. The first, a partnership with Clifford Woolf at Boston Children’s Hospital, was exploring how pain stimuli in the skin, joints, and bone are propagated into the spinal cord and conveyed to the brain, and where in the brain those signals go.
The second was a prestigious R35 grant, sometimes called the Outstanding Investigator Award, which provides flexible, long-term funding to established investigators to allow them to pursue particularly innovative research. It was meant to cover the bulk of Ginty’s work for eight years, but it was eliminated just one year in.
The most devastating part of the cancellations, he said, is that they come at a time of unparalleled progress in neurobiology.
“The advances are just breathtaking because of the alchemy of bringing together genetics and physiology and molecular biology, the knowledge that is being unveiled. At no other time in history have the advances been so rapid and so large as the time we’re in now. I feel fortunate to be in this position and to play a part in discovering how the nervous system works and new therapeutic opportunities. We need to find ways to survive the current funding crisis so that progress that leads to new treatments for disorders of the nervous system can continue.”