Cell therapy has emerged as a transformative approach to treating a range of diseases, including cancers, autoimmune disorders and genetic conditions. It achieves this by harnessing living cells, such as T cells, stem cells and engineered immune cells, to repair, replace or enhance biological functions.
Its potential to address previously untreatable conditions has triggered massive growth in the cell therapy market. Technological innovations in equipment and data analytics are critical for advancing cell therapy. These tools enhance efficacy, safety and scalability, creating a synergy between biological sciences and engineering (summarized in Figure 1). By refining production processes and optimizing protocols, these technologies bridge the gap between laboratory research, therapy manufacturing and product QC application.
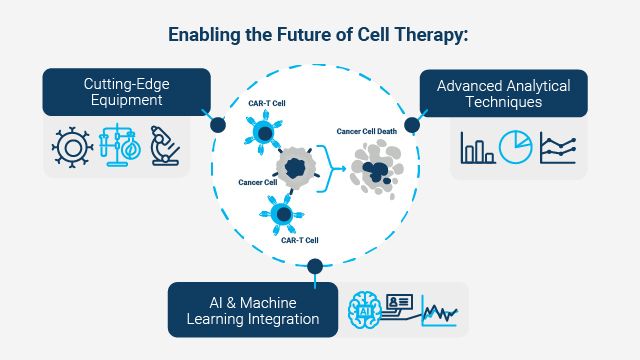
Figure 1: Key aspects that will play a part in advancing cell therapies. Credit: Agilent Technologies.
Cutting-edge equipment in cell therapy
Bioreactors provide controlled environments essential for the growth and differentiation of therapeutic cells (e.g. chimeric antigen receptor (CAR) T, stem cells) under good manufacturing practice (GMP) conditions, enabling the transition from small-scale laboratory research to large-scale production. These systems ensure consistency and scalability while maintaining the viability, integrity, phenotype and function of cell products.
Significantly, the development of single-use and closed bioreactors has reduced contamination risks and streamlined processes. Perfusion-based bioreactors support long-term, high density cell cultures. Integrating reactors with automated real-time monitoring systems enhances control over parameters such as pH, temperature and oxygen levels, ensuring optimal cell growth conditions.
Cell sorters enable the isolation of specific cell populations based on markers such as surface proteins and/or viability indicators. Techniques like fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS) are commonly used, relying on fluorescent labeling or magnetic tagging. These techniques play a critical role in cell therapy development and production and are being used for the selection of desired therapeutics cell subs-sets (e.g., regulatory T cells, naïve T cells) and removal of undesired cells (e.g., dead cells, non-T lymphocyte leukocytes) to enhance cell therapy product purity, potency and safety.
Recent innovations include microfluidic-based sorting for gentle cell separation, close-system sorters, enhanced and improved FACS sorting platforms for automated cell therapy platforms. These advances have improved the speed and accuracy of isolating rare cell populations, ensuring GMP-compliant, high-purity cell products with minimal manual handing.
CRISPR-Cas9 technology enables precise genome editing, allowing researchers to correct genetic mutations (e.g. PD-1 knockout to disrupt immune evasion pathways in cancer), enhance the functionality of therapeutic cells (e.g. engineering CAR T cells) or create universal donor cells by eliminating human leukocyte antigen (HLA) expression. Recent developments include novel delivery methods, such as lipid nanoparticles and electroporation, for safer gene transfer, as well as base and prime editing techniques that reduced off-target effects and improved precision. These advances in CRISPR are transforming cell therapy from a reactive approach to a programable one, creating next-generation cell therapies with enhanced safety and efficacy.
Advanced analytical techniques for cell therapies
Flow cytometry and cytometry by time-of-flight (CyTOF) have revolutionized immunophenotyping by enabling high-dimensional analysis of cell surface markers. Recent developments in spectral flow cytometry and AI-driven, cloud-based platforms for conventional or spectral cytometry data analysis, have made high dimensional cytometry (e.g., > 40 markers) widely accessible to scientists and developers in cell therapy laboratories. New applications include high-resolution profiling of immune and tumor microenvironments, helping to decipher the cellular mechanisms of cancer and therapy efficacy, and mapping immune landscapes in individuals to provide insights into disease progression and therapeutic response.
Advanced robotic systems and microfluidic devices have automated the screening of large libraries of engineered cells. One example is polyethylene glycol (PEG)-based nanovials with inner cavities selectively coated with biotinylated gelatin, enabling high-throughput screening and sorting of mesenchymal stem cells with enhanced extracellular vesicle production. These technologies accelerate the discovery of therapeutic candidates, including novel CAR T-cell constructs.
Single-cell sequencing provides high-resolution insights into gene expression, epigenetics and the genome at the individual cell level, uncovering critical information about cell function and therapeutic efficacy. New developments include multiomics platforms that integrate RNA sequencing with epigenetic profiling or analyze gene expression alongside immune receptor repertoires to profile immune cell diversity. Additionally, spatial transcriptomics enables gene expression in tissue contexts. These innovations have led to the identification of novel targets for CAR T-cell therapy, highlighting the transformative potential of single-cell sequencing.
The power of data analytics for cell therapy
AI and machine learning are increasingly used to analyze and interpret complex biological and manufacturing data. Applications include:
- Integrating omics, imaging and clinical data to guide therapy development decisions
- Identifying critical quality attributes (CQAs) for manufacturing optimization
- Developing predictive biomarkers for therapy selection and individual prognosis
In CAR T-cell therapy, for instance, machine learning models have improved target antigen selection and minimized off-target effects.
Combining genomics, proteomics and metabolomics provides a holistic understanding of cell function. Multiomics approaches personalize treatments based on individual’s profiles, advancing cancer research and therapeutic design. Recent studies have applied multiomics to tumor subtyping, prognosis and diagnosis, enhancing the understanding of cancer biology and aiding in the discovery of new therapeutic targets.
Potency assays and cell therapy characterization
Potency assays measure the biological activities of cell products, ensuring they function according to their intended mechanism of action and meet therapeutic standards. Ongoing standardization efforts have streamlined regulatory approval processes and improved the reliability of cell-based therapies.
Validated potency assays have become essential for applications in CAR T therapy, cardiac regenerative medicine and other therapeutic areas, providing critical benchmarks for efficacy and safety.
Recent advancements
- Machine learning models can help predict cytokine release syndrome, a common side effect of CAR T-cell therapy. These tools enable pre-emptive interventions, improving health outcomes.
- Real time cell analysis (RTCA) has been adopted for CAR T-cell potency assessment by continuously monitoring immune cell killing of target cancer cells. The RTCA technology enhances the accuracy, reliability and precision of the potency analytical procedure.
- The advent of off-the-shelf allogeneic therapies, which use donor-derived rather than individually sourced cells, addresses scalability challenges and reduces treatment costs. Recent advances in this area promise broader accessibility and faster delivery of cell therapies.
The future of cell therapy
The integration of advanced equipment and data analytics is transforming the field of cell therapy. Innovations in bioreactors, genome editing and analytical tools are enhancing both precision and scalability, while AI and multiomics approaches are optimizing therapeutic protocols.
As these technologies continue to evolve, cell therapy is poised to deliver more effective, personalized treatments. These advances hold the promise of addressing unmet medical needs and revolutionizing healthcare.